Explore the effects of Ozempic on weight management.
January 10, 2025
Written by Turian Biel

Table of Contents
- Understanding Ozempic's Impact on Weight Loss
- Ozempic's Role in Weight Management
- Sales Growth of Weight Loss Medications
- Patient Experiences with Ozempic
- Health Risks Associated with Ozempic
- Ozempic's Effectiveness Compared to Other Medications
- Counterfeit Ozempic Concerns
- Public Perception and Demand for Ozempic
- Long-Term Effects of Ozempic Use
- Regulatory Status of Ozempic
- Exploring the Side Effects of Ozempic
- Future Trends in Weight Loss Medications
- Conclusion
Understanding Ozempic's Impact on Weight Loss
Ozempic's Role in Weight Management
Ozempic, which contains the active ingredient semaglutide, is primarily used for treating type 2 diabetes but has gained popularity for weight loss. Studies indicate that patients can experience an average weight loss of up to 15% of their body weight over a period of 68 weeks. This medication works by mimicking GLP-1, which regulates appetite and glucose levels, leading to reduced food intake.
Sales Growth of Weight Loss Medications
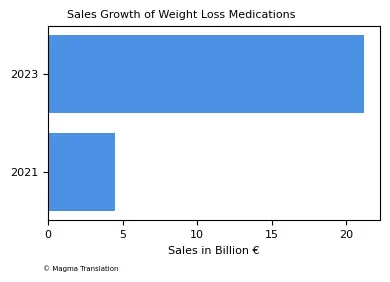
The demand for weight loss medications, including Ozempic, has surged significantly. Reports show that sales of these medications increased by 92% this year, reflecting a growing trend in the pharmaceutical industry. The sales figures rose from €4.5 billion in 2021 to €21.2 billion in 2023, indicating a strong market for these treatments.
Patient Experiences with Ozempic
Many users of Ozempic report significant changes in their eating habits. Patients have noted a decrease in hunger and a reduction in the number of meals consumed daily. However, rapid weight loss can lead to undesirable side effects, such as the phenomenon known as "Ozempic face", which causes sagging and wrinkles due to the loss of facial fat.
Health Risks Associated with Ozempic
While Ozempic can be effective for weight loss, it is not without risks. Users may experience side effects such as gastrointestinal issues, including nausea and diarrhea. A recent case involved Cuban actress Lisandra Silva, who was hospitalized after experiencing severe drops in blood sugar levels due to Ozempic use, highlighting the potential dangers of this medication source.
Ozempic's Effectiveness Compared to Other Medications
In clinical studies, Ozempic has shown promising results compared to other weight loss medications. For instance, a study indicated that patients using Mounjaro, another GLP-1 receptor agonist, achieved greater weight loss results than those on Ozempic. Approximately 82% of Mounjaro users lost 5% of their initial weight after one year, compared to 67% of Ozempic users.
Counterfeit Ozempic Concerns
The World Health Organization (WHO) has issued alerts regarding counterfeit Ozempic products, which have been detected in various countries, including Brazil and the United States. The use of counterfeit medications poses serious health risks, as they may contain incorrect dosages or harmful substances source.
Public Perception and Demand for Ozempic
Public interest in Ozempic has surged, with many individuals seeking it for weight loss despite its primary indication for diabetes management. The term "Ozempic face" has emerged on social media, referring to facial changes resulting from rapid weight loss associated with the drug. This phenomenon has gained attention on platforms like TikTok, where videos showcasing this effect have gone viral source.
Long-Term Effects of Ozempic Use
Long-term use of Ozempic raises questions about sustainability in weight management. Studies have shown that while patients can achieve significant weight loss, many regain weight after discontinuing the medication. Research indicates that approximately 67% of the weight lost is regained after stopping treatment, emphasizing the need for ongoing lifestyle changes.
Regulatory Status of Ozempic
Ozempic is approved for use in several countries, including Brazil, where it is indicated for adults with obesity or overweight. However, its use for weight loss is considered off-label, raising concerns about the need for medical supervision. The Brazilian Health Regulatory Agency (Anvisa) has approved Wegovy, a higher dosage version of Ozempic, specifically for obesity treatment source.
Exploring the Side Effects of Ozempic
Common Side Effects of Ozempic
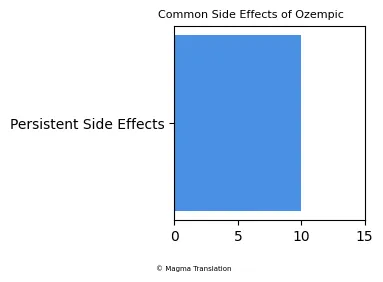
Patients using Ozempic may experience various side effects, with gastrointestinal issues being the most common. Reports indicate that users often face symptoms such as nausea, vomiting, and diarrhea. These side effects are generally mild to moderate but can lead to discontinuation of the medication in some cases. A study highlighted that approximately 10% of users may experience persistent side effects that affect their ability to tolerate the drug.
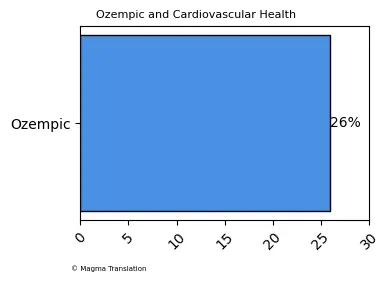
Ozempic and Cardiovascular Health
Ozempic has been shown to have positive effects on cardiovascular health, particularly for patients with type 2 diabetes. Research indicates that semaglutide can reduce the risk of serious cardiovascular events, such as heart attacks and strokes. A clinical trial demonstrated that patients using Ozempic had a 26% reduction in the risk of major adverse cardiovascular events compared to those on placebo, making it a valuable option for at-risk individuals.
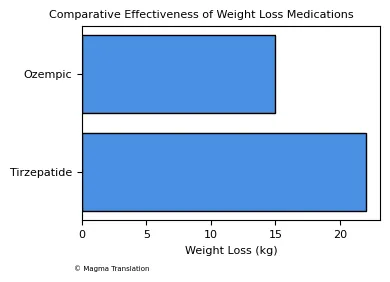
Comparative Effectiveness of Weight Loss Medications
When comparing Ozempic to other weight loss medications, it is essential to consider their effectiveness. Studies have shown that tirzepatide, the active ingredient in Mounjaro, may lead to greater weight loss than semaglutide. In clinical trials, patients using tirzepatide lost an average of 22 kg, while those on Ozempic lost around 15 kg, indicating a significant difference in outcomes.
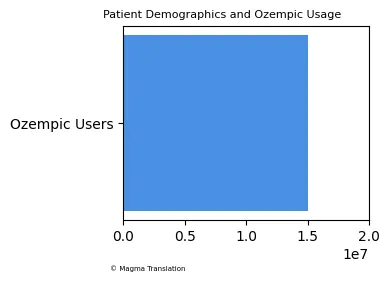
Patient Demographics and Ozempic Usage
The demographics of patients using Ozempic for weight loss are diverse, with a notable increase in interest among younger adults. A recent survey indicated that more than 15 million Americans reported using weight loss medications, including Ozempic, highlighting a growing trend among various age groups seeking effective solutions for obesity.
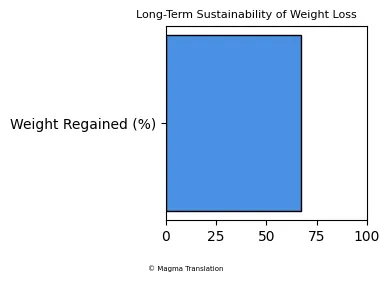
Long-Term Sustainability of Weight Loss with Ozempic
While Ozempic can lead to significant weight loss, questions remain about the sustainability of these results. Research suggests that many patients regain weight after discontinuing the medication. A study found that approximately 67% of the weight lost is regained within a year of stopping treatment, emphasizing the importance of lifestyle changes alongside medication.
Insurance Coverage and Accessibility of Ozempic
Access to Ozempic can vary significantly based on insurance coverage and healthcare policies. In some regions, patients may face high out-of-pocket costs, with prices reaching R$ 1,000 for a monthly supply. This financial barrier can limit access for many individuals who could benefit from the medication, raising concerns about equitable healthcare access.
Public Health Implications of Ozempic Use
The increasing use of Ozempic for weight loss has significant public health implications. As obesity rates continue to rise globally, the demand for effective treatments like Ozempic is likely to grow. However, the off-label use of diabetes medications for weight loss raises ethical concerns and highlights the need for proper medical supervision to prevent misuse and potential health risks source.
Future Trends in Weight Loss Medications
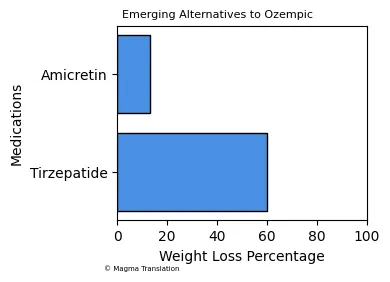
Emerging Alternatives to Ozempic
As the demand for weight loss medications continues to rise, new alternatives to Ozempic are being developed. Medications like tirzepatide and amicretin are gaining attention for their potential effectiveness in weight management. Recent studies indicate that tirzepatide may lead to a weight loss of up to 60% over eight months, while amicretin has shown promise with a 13% reduction in weight over four months. These emerging treatments may provide additional options for individuals struggling with obesity.
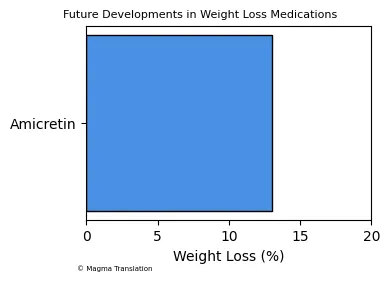
Future Developments in Weight Loss Medications
As the demand for effective weight loss solutions continues to rise, pharmaceutical companies are investing in the development of new medications. Innovations in GLP-1 receptor agonists, such as the upcoming drug amicretin, promise to deliver significant weight loss results. Early studies suggest that amicretin could lead to a reduction of 13% of body weight in just 4 months. This ongoing research highlights the potential for more effective treatments in the future, addressing the growing obesity epidemic.
Conclusion
In summary, Ozempic has emerged as a significant player in the weight loss medication landscape, offering promising results for many patients. However, its use comes with risks and considerations that must be addressed through proper medical supervision and ongoing research.
